David Colquhoun
There’s no remedy for the Prince of Quacks
This is the title of a piece by Francis Wheen in the London Evening Standard, 16 May 2006. Francis Wheen is the author of the Top ten delusions.
“Prince Charles travels to Geneva next week to deliver the keynote speech at the annual assembly of the World Health Organisation. Some mistake, surely?” “The WHO describes Charles as the president of the Prince’s Foundation for Integrated Health and “patron of a number of health charities”. It omits to add that his views on medicine are barmy – and pernicious. ”
“WHO delegates from 192 nations have plenty to discuss during their five-day meeting – HIV/Aids, sickle-cell anaemia, preparations for a flu pandemic, the eradication of polio and smallpox. Why waste precious time listening to the heir to the British throne, who has spent more than 20 years displaying his ignorance of medical science?”
“The prince has never met a snake oil vendor he didn’t like. A couple of years ago he urged doctors to prescribe coffee enemas to cancer patients, a suggestion which provoked this rebuke from Professor Michael Baum of University College London: “The power of my authority comes with a knowledge built on 40 years of study and 25 years of active involvement in cancer research. Your power and authority rest on an accident of birth.” ”
The Prince’s Foundation for Integrated Health publishes Complementary healthcare: a guide for patients which is full of wishful thinking. For example, it tells the unfortunate patient that
“Homeopathy is most often used to treat chronic conditions such as asthma; eczema; arthritis; fatigue disorders like ME; headache and migraine; menstrual and menopausal problems; irritable bowel syndrome; Crohn’s disease; allergies; repeated ear, nose, throat and chest infections or urine infections; depression and anxiety.”
but says nothing at all about whether or not they work. That is just irresponsible. And to describe pills that contain no trace of the substance on the label as ”very diluted” is plain dishonest .
This item was transferred from the old IMPROBABLE SCIENCE page.
This is a topic that I have kept well away from, because I have an obvious vested interest: “no pipe, no algebra”. But the topic does make an interesting example of the effect of political correctness on people who are otherwise impeccable in there attitude to evidence. Tim Luckhurst writes about this in The Independent (2 May, 2006).
“On Desert Island Discs in 2001, Sir Richard Doll, the man who proved the incontrovertible causal link between active smoking and lung cancer, said: “The effect of other people smoking in my presence is so small it doesn’t worry me.”
He was right not to fret. One of the largest studies of the health consequences of secondary smoking was published in the British Medical Journal in 2003. It tracked the health of 118,000 Californians over four decades in a rigorous attempt to identify a causal relationship between environmental tobacco smoke (the scientific term for secondary smoke) and premature death. It concluded: “The results do not support a causal relationship between ETS and tobacco-related mortality.” ”
The paper in question is ‘Environmental tobacco smoke and tobacco related mortality in a prospective study of Californians, 1960-98’, James E Enstrom and Geoffrey C Kabat 2003, BMJ , 326, 1057 . The publication was followed by a torrent of abuse, more
reminiscent of religious zealotry than of science. The responses have been analysed in an article in Public Understanding of Science (2005, 14, 5–23) by Ungar and Bray, ‘Silencing science: partisanship and the career of a publication disputing the dangers of secondhand smoke’ [ download pdf ].
I don’t know what the final answer will be about the risks of passive smoking, but as a pharmacologist, the higher levels of damage reported seem barely credible, bearing in mind that
“Reputable research shows that a non-smoker inhales between a 500th and 1,000th of the toxins inhaled by the smoker himself.”
It does seem that it is not only big drug companies, and deluded homeopaths, who are happy to distort evidence for their own purposes. Well-meaning zealots can do it too. That is just as scary.
“Isambard Kingdom Brunel’s 40-a-day cigar habit is held responsible for some of the greatest triumphs of British engineering. Unfortunately, it also represents an upturned middle finger towards the politically-correct mandarins of modern academia. With this in mind, Brunel University has removed the famous stoogie from a new, life-size statue of the eminent Victorian. The bronze is based on the National Portrait Gallery’s iconic photograph of Brunel standing next to the launching chains of his ship, the SS Great Eastern, in 1857. It was unveiled last week, revealing a close likeness, but – to the annoyance of Brunel fans, historians and the smoking lobby alike – no cigar.” The Independent , 18th July 2006.
Some scientific heros. Their longevity tells you absolutely nothing.


Transferred from the original IMPROBABLE SCIENCE page.
Follow-up
An Edinburgh hospital is to supply aromatherapy on the NHS.
Read full entry on the original IMPROBABLE SCIENCE page.
A bizarre organisation called the NHS Trusts Association promotes not only homeopathy, but even wackier things like ‘crystal therapy’.
Read full entry on the original IMPROBABLE SCIENCE page.
The Amsterdam Medical Disciplinary Tribunal has struck off one doctor and suspended two others for their exclusive use of complementary treatments, resulting in the death of a woman.
Read full entry on the original IMPROBABLE SCIENCE page.
Trust Boots
Boots the Chemists (now Alliance Boots) is a very big business in the UK. There have 1,450 pharmacies in the UK and employ over 100,000 people.
I posted the item below a while ago, on the old Improbable Science page. I thought it deserved a bit more publicity, for the following reason. The quackometer has posted about Boots too,
I mentioned it during the debate with Felicity Lee at the British Pharmaceutical Conference (2007) (Ben Goldacre’s interview with Felicity Lee is a gem). After the talk I was approached by two heavies. Well, two men in dark suits anyway. It turned out that one was from Boots and the other from Alliance Pharmacies, now merged to form Boots Alliance. They seemed rather bothered by the fact that I’d criticised Boots, but were not entirely unreasonable. They claimed to be on the scientific side and said they’d investigate the matter. I wrote to the Boots man on 10 September, but got no reply, After a reminder on 29 October, I got this.
| Dear David Thank you for your email and reminder. We have investigated the points you raised in your blog. I was informed that it was an old leaflet and has not been reprinted (to my knowledge). However on a point of principle, I have raised the wider issue of clinical validity in my department. This will take its course through to the commercial/buying team. |
| Dear David Thank you for pointing this out. I’ve had a quick look and it is an educational website looking at all aspects of medicine and therapy, including alternative medicine. It is not a direct sales message to the public. I hope this helps |
Corporate Social Responsibility
Boots web site makes a big point about Corporate Social Responsibility (CSR)
“TRUST BOOTS
As you may have noticed, that’s the tagline which in 2005 we adopted as the sign-off to all our advertising. But it’s much more than just a slogan. It’s a concise statement of our entire corporate strategy. Our aim is to make Boots the world’s best health and beauty retailer, and we’re 100% clear that the unique trust in which we are held provides the key to achieving this. Which means, of course, that those two words are also the rationale for all our CSR activities. Everything we do that builds trust is good for our business; anything which could compromise it, a risk we can’t afford to take.”
Trust Boots to provide straight answers.
At one time. Boots were sufficiently ethical not to deal in homeopathy. But no longer.
When asked for evidence that the things they sell actually work, the Boots help desk is astonishingly coy, as related here (thanks to ebm-first.com for giving publicity to this report).
When Boots were asked about their ‘Alternatives Hayfever Relief Tablets’, the answer came, after some delay, “This is a homeopathic product, further information on homeopathic products is available from the Nelson company who make this
particular product for Boots. ” This company has been making homeopathic products for many years and
may well be able to help you further. You may also find general
information about homeopathic medicines in reference books in the public
library”. The email address that they gave me for Nelson’s did not work, and writing to another Nelson’s address produced no reply at all. Clearly any letter that contains the word “evidence” arouses suspicion and is simply deflected.
Dangerous advice from Boots: a small sting.
I have been into several Boots stores, sought out the most senior pharmacist that I can find, and asked them the following question. “I have a 5 year old son who has had diarrhoea for three days now. Please can you recommend a natural remedy”. The response was interesting. In every case but one, the pharmacist reached for a copy of the Boots pamphlet on homeopathy, and thumbed through it, while desperately, but unsuccessfuly, trying to retain an air of professional authority. Then one or another homeopathic treatment from the booklet was recommended. In only one case out of six did the pharmacist even mention the right answer (GP and rehydration). One pharmacist, who turned out to have qualified in Germany, was very insistent that homeopathic treatment was inappropriate and that I should should start rehydration and take the child to the GP. The other five, including one who had an impressive-looking badge saying “consultant pharmacist”, did not even mention rehydration.
Conclusion The education of the pharmacists was clearly insufficient for them to give reliable advice. On the contrary, their advice was downright dangerous.
Miseducation by Boots the chemists
Boots also run an “educational” web site for children, the ‘Boots learning store’. Click on the section for ‘pupils’, and then ’16+’ and you find their education about alternative medicine (do their pharmacists do this course, I wonder?). The slide show that follows is an insult to human intelligence,
|
 |
Then follows a totally misleading slide about enzymes.
There is nothing wrong with the enzyme bit, but the analogy with homeopathy is baseless and misleading. Enzymes don’t work when there are no molecules present.
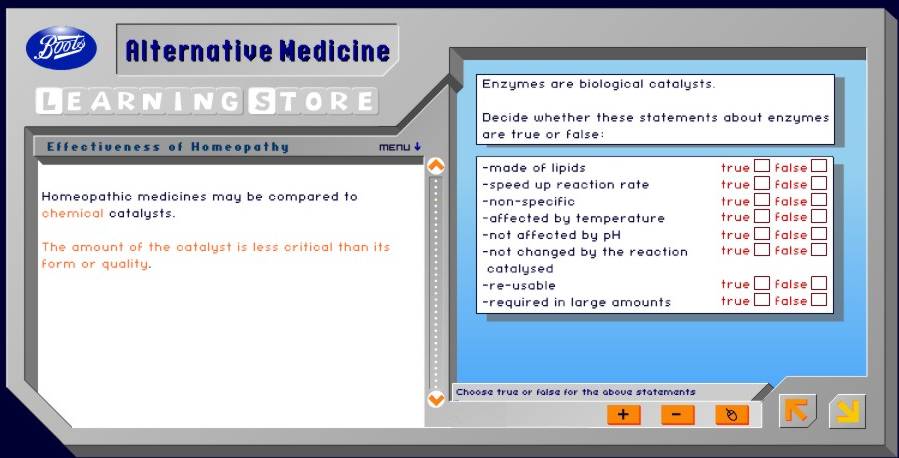
But in the next slide, enzymes and catalysts are forgotten anyway, This is how it works.

This meaningless mediaeval gobbledygook about ‘vital forces’ is being peddled as ‘education’ by the biggest retail pharmacy chain in the UK. What hope is there for kids?
But there is more. Now for the exam. If you click on the ‘teacher’ section you can download the students’ notes and the test. The ‘Student Notes’ include the following direct claim that homeopathy can cure diseases.

Now take the test, Here is question 1, and the answer.


I suppose that if the educators at Boots classify Hahnemann’s provings as a ‘clinical trial’ it goes a long way to explain the quality of their learning store, and the quality of the advice given by their pharmacists.
Boots Alternatives also sells a “snoring remedy”
The evidence for effectiveness of this herbal product is very dodgy, as described here earlier. This was an interesting saga that involved bad statistics, inappropriate controls and concealed financial interests. It eventually appeared on the BBC Radio 4 programme, You and Yours.
Postcript: “Nurses and pharmacists are to be given greater powers to prescribe drugs”
The foregoing history does not give one much confidence in the government’s latest money-saving wheeze. [BBC]
“The latest measures mean nurses and pharmacists will be able to prescribe treatments for more serious conditions such as heart disease and diabetes – traditionally the domain of GPs.
Health Secretary Patricia Hewitt said: “Nurse and pharmacist independent prescribing is a huge step forward in improving patient accessibility to medicines from highly skilled and well trained staff.”
And Chief Pharmaceutical Officer Dr Keith Ridge added: “For pharmacists, this is the dawn of a new era. It will help transform the public’s perception of pharmacy and the services they deliver to patients.”
This item was first posted on the original IMPROBABLE SCIENCE page.
Here is a link to a fascinating collection of essays that discuss “disease mongering”.
Read full entry on the original IMPROBABLE SCIENCE page.
Well this beats everything. Following the advice of the PPA, I re-submitted my request under the Freedom of Information Act to the Department of Health (DoH), which is where the PPA claimed to to have sent very single bit of information concerning their decision to make magnetic ulcer treatment available on the NHS.
When I mananged to decode the result (the DoH seem incapable of sending legible emails) this is what they said.
|
As you are aware, the decision by the PPA to allow Magnopulse;s 4Ulcercare on Part IX of the Drug Tarriff is a recent one and the documentation that went along with the application are still very much restricted as “commercially sensitive”. We have therefore decided to withhold this information under the FOI Act.
Section 43: Commercial Interests |
Trade secrets in magnets? They are one of the oldest scams of the health fraud industry! Are the DoH really so enthusiastic to protect these non-existent trade secrets? (The company whose trade secrets the DoH are so eager to protect are under invesitgation by the Office of Fair Trading!) Or is the DoH merely colluding to cover up the cockup at the PPA?
Either way, the Freedom of Information Act 2000 has yet to prove it’s worth the paper it’s written on.
Why not write to your MP to ask them to approach the DoH about this absurd misuse of the Act?
I have requested an internal investigation by the DoH. Next the appeal goes to the Ombudsman.
In the discussion of magnets on the Badscience site, a Michael King says that 4ulcercare will be included in Part IX of the Drug Tariff because it meets the criteria of the Prescription Pricing Authority (PPA).
I presume this Michael King is Director of Planning and Corporate Affairs at the PPA, though he does not say so.
Michael King says
?There is no judgement offered about whether a product in the Drug Tariff
is more (or less) efficacious than any other, or the placebo effect.?
The criteria for inclusion in
Part IX of the Drug Tariff () include, in section 10 iii, ?They are cost
effective?
Will he please explain how a device can be cost-effective, if it is ineffective (relative to placebo)?
What the PPA says
Michael King has replied to my question by email (1 Mar 2006). He says
“The cost-effectiveness threshold for inclusion in the Drug Tariff is met if the ‘effectiveness’ of the device, as seen in data submitted by the manufacturer in support of the application, exceeds its cost to the NHS. ”
Sadly this is still ambiguous. It seems to suggest that that whatever data
are submitted by the manufacturer are taken at face value, without any attempt
to evaluate their quality. So I phoned King to ask if this was the case. He
was helpful, but he said that it was not the case. He told me that
the data were subject
to some sort of low level evaluation, short of the sort of evaluation that
NICE would do. This seems to contradict his earlier statement (above) that
inclusion in the Tariff implies no judgement about whether a device is better
than a placebo.
King said also that listing in the Tariff
“. . . is not a licensing decision nor a recommendation akin to the outcome of a NICE review”
The problem is, of course, that listing is seen as a recommendation by the public, by the Daily
Mail, and certainly by the manufacturer.
One thing, at least, is clear in this case. Whatever evaluation was done,
it was done very badly. But in order to try to find out exactly what evaluation
was done, and by whom, I’m having to resort to the Freedom of Information Act.
Watch this space.
What NICE says
Fraser Woodward (Communications Manager, National Institute for Health and Clinical Excellence (NICE)) writes as follows.
“The test of “cost effectiveness” applied by the PPA when determining whether or not a device should go on the tariff is very different to the way cost effectiviness is assessed by NICE”
That is pretty obvious, but how is the public meant to know that, when they hear that the NHS has declared a treatment to be ‘cost-effective’, that statement can mean two entirely different things according to which part of the bureaucracy the statement comes from?
Several of the people who contributed to, and/or appeared in, the BBC2 series on alternative medicine, have complained that they were treated “like marionettesâ€, and that the programme was sensationalised and uncritical,
Read full entry on the original IMPROBABLE SCIENCE page.
The Office of Fair Trading has taken Magno-Pulse Ltd to the High Court after they refused to stop what the OFT regards, quit rightly, as misleading advertising. This is the company whose magnets have just been approved by the PPA for prescription on the NHS!
Read full entry on the original IMPROBABLE SCIENCE page.
Emails in my possession show that the Chiron Clinic is able to decide that nutritional supplements are needed for leg ulcers on the basis of an email (as well as magnets of course).
Read full entry on the original IMPROBABLE SCIENCE page.
In the discussion of magnets on the Badscience site, a Michael King says that 4ulcercare will be included in Part IX of the Drug Tariff because it meets the criteria of the Prescription Pricing Authority (PPA)
.
I presume this Michael King is Director of Planning and Corporate Affairs at the PPA, though he does not say so.
Michael King says
?There is no judgement offered about whether a product in the Drug Tariff
is more (or less) efficacious than any other, or the placebo effect.?
The criteria for inclusion in
Part IX of the Drug Tariff () include, in section 10 iii, ?They are cost
effective?
Will he please explain how a device can be cost-effective, if it is ineffective (relative to placebo)?
What the PPA says
Michael King has replied to my question by email (1 Mar 2006). He says
“The cost-effectiveness threshold for inclusion in the Drug Tariff is met if the ‘effectiveness’ of the device, as seen in data submitted by the manufacturer in support of the application, exceeds its cost to the NHS. ”
Sadly this is still ambiguous. It seems to suggest that that whatever data
are submitted by the manufacturer are taken at face value, without any attempt
to evaluate their quality. So I phoned King to ask if this was the case. He
was helpful, but he said that it was not the case. He told me that
the data were subject
to some sort of low level evaluation, short of the sort of evaluation that
NICE would do. This seems to contradict his earlier statement (above) that
inclusion in the Tariff implies no judgement about whether a device is better
than a placebo.
King said also that listing in the Tariff
“. . . is not a licensing decision nor a recommendation akin to the outcome of a NICE review”
The problem is, of course, that listing is seen as a recommendation by the public, by the Daily
Mail, and certainly by the manufacturer.
One thing, at least, is clear in this case. Whatever evaluation was done,
it was done very badly. But in order to try to find out exactly what evaluation
was done, and by whom, I’m having to resort to the Freedom of Information Act.
Watch this space.
What NICE says
Fraser Woodward (Communications Manager, National Institute for Health and Clinical Excellence (NICE)) writes as follows.
“The test of “cost effectiveness” applied by the PPA when determining whether or not a device should go on the tariff is very different to the way cost effectiviness is assessed by NICE”
That is pretty obvious, but how is the public meant to know that, when they hear that the NHS has declared a treatment to be ‘cost-effective’, that statement can mean two entirely different things according to which part of the bureaucracy the statement comes from?
Nobody knows the cost! But here is some information that I found by use of the Freedom of Information Act 2000…
Read full entry on the original IMPROBABLE SCIENCE page.
[This post was transferred from the old Improbable Science page]
It’s not just homeopathy. The Sunday Times, (26 February, 2006) reports that the National Health Service has fallen for another scam.
“IT COULD be called the Cleopatra Effect. Magnetic therapy, which has held the rich and powerful in thrall from ancient Egypt to modern Downing Street, is about to be made available on the National Health Service.
NHS accountants are so impressed by the cost-effectiveness of a “magnetic leg wrap” called 4UlcerCare that from Wednesday doctors will be allowed to prescribe it to patients.”
This is nicely timed to coincide with an Editorial in the British Medical Journal, by Finegold & Flamm (2006) (click to download pdf file). The editorial title was “Magnet therapy. Extraordinary claims, but no proved benefits“. They conclude
“Patients should be advised that magnet therapy has no proven benefits. If they insist on using a magnetic device, they could be advised to buy the cheapest – this will alleviate the pain in their wallet,”
For example, Carter et al,, 2002 found no detectable effect of magnet therapy for treatment of wrist pain Attributed to Carpal Tunnel Syndrome (30 patients,double blind, careful controls). Winemiller et al., 2003 (Journal of American Medical Association, 290, 1474–78), found no benefit of magnets vs sham-magnets in treatment of plantar heel pain in 101 patients.
Magnets are said to be one of Cherie Blair’s several curious and irrational beliefs. It is alleged, according to the Daily Telegraph, that “Cherie Blair did not allow her youngest child, Leo, to have the controversial MMR vaccine and instead asked a New Age healer to wave a “magic” pendulum over him”. A few more examples are documented here and here.
The accountants at the Prescription Pricing Authority have decided that the “the magnets will save money on bandages and nurses’ time by healing the wounds.” I dare say they could save even more money by removing all effective treatments.
The evidence in favour of the magnetic treatment all seems to come from a Dr Nyjon Eccles. The Sunday Times describes him as an “NHS GP in north London”, but elsewhere he is described as "Founder, CEO and Medical Director of the Chiron Clinic" in Harley Street. A look at their web site shows that they offer a full range of alternative scams. Cancer patients can get
“LYMPH DETOXIFICATION – This is achieved by non-invasive scalar, oxygen-fed light beam therapy. This helps to detoxify the tissues by assisting the body in dissolving lymph blockages and restoring normal lymph flow using the Nobel quantum scalar technology coupled with oxygen for remarkable healing potential.”
This is total gobbledygook, designed to take advantage of the desperate.
The only real evidence to be provided by Dr Eccles that the device works is a small (26 patients) double blind trial that has not yet been published in a peer-reviewed journal, and which suffered from a number of problems (dropouts, outliers). What, I wonder, does NICE think of evidence like this?
More on magnets and the PPA
In the discussion of magnets on the Badscience site, a Michael King says that 4ulcercare will be included in Part IX of the Drug Tariff because it meets the criteria of the Prescription Pricing Authority (PPA).
I presume this Michael King is Director of Planning and Corporate Affairs at the PPA, though he does not say so.
Michael King says
“There is no judgement offered about whether a product in the Drug Tariff
is more (or less) efficacious than any other, or the placebo effect.”
The criteria for inclusion in
Part IX of the Drug Tariff () include, in section 10 iii, “They are cost effective”
Will he please explain how a device can be cost-effective, if it is ineffective (relative to placebo)?
What the PPA says
Michael King has replied to my question by email (1 Mar 2006). He says
“The cost-effectiveness threshold for inclusion in the Drug Tariff is met if the ‘effectiveness’ of the device, as seen in data submitted by the manufacturer in support of the application, exceeds its cost to the NHS. ”
Sadly this is still ambiguous. It seems to suggest that that whatever data are submitted by the manufacturer are taken at face value, without any attempt to evaluate their quality. So I phoned King to ask if this was the case. He was helpful, but he said that it was not the case. He told me that the data were subject to some sort of low level evaluation, short of the sort of evaluation that NICE would do. This seems to contradict his earlier statement (above) that inclusion in the Tariff implies no judgement about whether a device is better than a placebo.
King said also that listing in the Tariff
“. . . is not a licensing decision nor a recommendation akin to the outcome of a NICE review”
The problem is, of course, that listing is seen as a recommendation by the public, by the Daily
Mail, and certainly by the manufacturer.
One thing, at least, is clear in this case. Whatever evaluation was done, it was done very badly. But in order to try to find out exactly what evaluation was done, and by whom, I’m having to resort to the Freedom of Information Act.
The results are here and here..
What NICE says
Fraser Woodward (Communications Manager, National Institute for Health and Clinical Excellence (NICE)) writes as follows.
“The test of “cost effectiveness” applied by the PPA when determining whether or not a device should go on the tariff is very different to the way cost effectiviness is assessed by NICE”
That is pretty obvious, but how is the public meant to know that, when they hear that the NHS has declared a treatment to be ‘cost-effective’, that statement can mean two entirely different things according to which part of the bureaucracy the statement comes from?

